Question Video: Calculating 𝐾_𝑝 at Equilibrium for a Mixture of Nitrogen, Hydrogen, and Ammonia | Nagwa
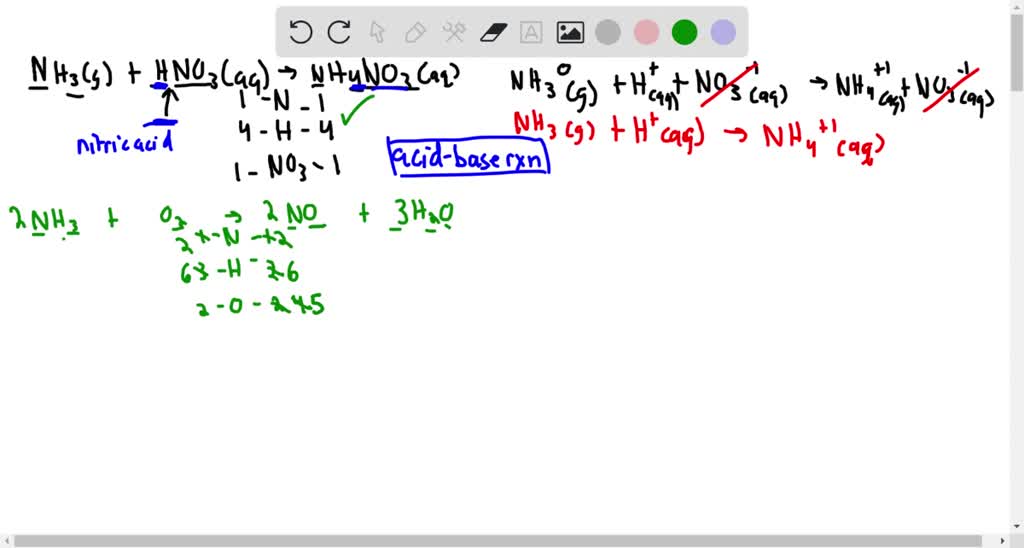
SOLVED:The fertilizer, ammonium nitrate, is made by reacting ammonia with nitric acid. (Section 1.4) (a) Write a balanced equation, with state symbols, for the reaction of ammonia gas with nitric acid to

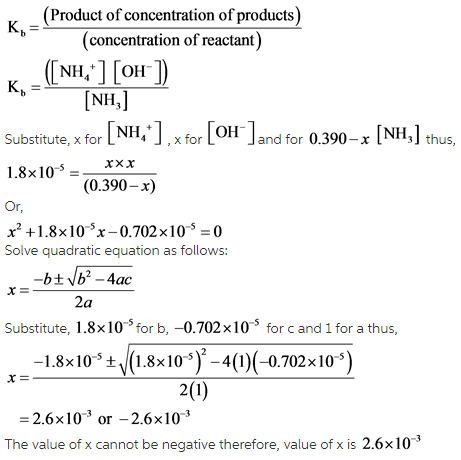
Calculate the pH of a 0.10 M ammonia solution . Calculate the pH after 50.0 mL of this solution is treated with 25.0 mL of 0.10 M HCl. The dissociation constant of ammonia, Kb=1.77xx10^(-5)

Step-by-step guide to calculating unionized (toxic) ammonia. UIA of... | Download Scientific Diagram

50 kg of nitrogen and 10 kg of hydrogen are mixed to produce ammonia. Calculate the ammonia formed and identify the limiting reagent in the production of ammonia in this situation.
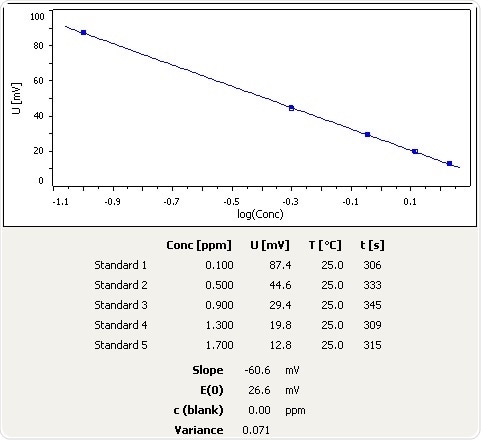
How to calculate your Un-ionized ammonia levels Information below collected from: Francis-Floyd, Ruth, Craig Watson, Denise Pett

Calculate the equilibrium constant (Kc) for the formation of NH3 in the following reaction: N2 (g) + 3H2 (g) 2NH3 (g) At equilibrium, the concentration of NH3, H2 and N2 are 1.2 ×

Reaction Mechanism and Kinetics for Ammonia Synthesis on the Fe(111) Surface | Journal of the American Chemical Society


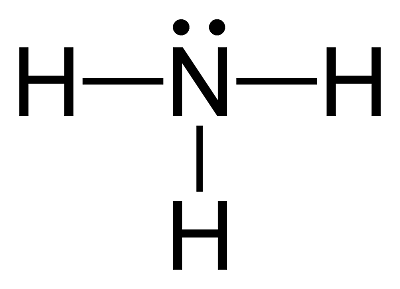

![Ammonia [NH3] Molecular Weight Calculation - Laboratory Notes Ammonia [NH3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/ammonia-molecular-weight-calculation-300x150.jpg)